Methicillin-resistant Staphylococcus aureus (MRSA) ( or ) is a gram-positive bacterium that is genetically different from other strains of Staphylococcus aureus. MRSA is responsible for several difficult-to-treat infections in humans. MRSA is any strain of S. aureus that has developed, through horizontal gene transfer and natural selection, multiple drug resistance to beta-lactam antibiotics. ?-lactam antibiotics are a broad spectrum group which includes some penams - penicillin derivatives such as methicillin and oxacillin, and cephems such as the cephalosporins. Strains unable to resist these antibiotics are classified as methicillin-susceptible Staphylococcus aureus, or MSSA.
MRSA is prevalent in hospitals, prisons, and nursing homes, where people with open wounds, invasive devices such as catheters, and weakened immune systems are at greater risk of nosocomial infection (hospital-acquired infection). MRSA began as a hospital-acquired infection, but has developed limited endemic status and is now community-acquired as well as livestock-acquired. The terms HA-MRSA (healthcare-associated or hospital-acquired MRSA), CA-MRSA (community-associated MRSA) and LA-MRSA (livestock-associated) reflect this distinction.
Video Methicillin-resistant Staphylococcus aureus
Microbiology
Like all Staphylococcus aureus (usually S. aureus but abbreviated SA at times), methicillin-resistant Staphylococcus aureus (MRSA) is a gram-positive, spherical (coccus) bacterium that is about 1 micron in diameter. It does not form spores and it is non-motile. It forms grape-like clusters or chains. Unlike Methicillin-susceptible Staphylococcus aureus MSSA, MRSA is slower growing on a variety of media and has been found to exist in mixed colonies of MSSA. The mecA gene, which confers the resistance to a number of antibiotics is present in MRSA and not in MSSA. In some instances, the mecA gene is present in MSSA but is not expressed. Polymerase chain reaction (PCR) testing is the most precise method in identifying MRSA strains. Specialized culture media have been developed to better differentiate between MSSA and MRSA and in some cases, it will identify specific strains that are resistant to different antibiotics. Other strains of S. aureus have emerged that are resistant to oxacillin, clindamycin, teicoplanin, and erythromycin. These resistant strains may or may not possess the mecA gene. S. aureus has also developed resistance to vancomycin (VRSA). One strain is only partially susceptible to vancomycin and is called vancomycin-intermediate S. aureus (VISA). GISA is a strain of resistant S. aureus and stands for glycopeptide-intermediate S. aureus and is less suspectible to vancomycin and teicoplanin. Resistance to antibiotics in S. aureus can be quantified. This done by determining the amount of the antibiotic in micrograms/milliliter must be used to inhibit growth. If S. aureus is inhibited at a concentration of vancomycin of less than or equal to 4 micrograms/milliliter, it is said to be susceptible. If a concentration of greater than 32 micrograms/milliliter is necessary to inhibit growth, it is said to be resistant.
Maps Methicillin-resistant Staphylococcus aureus
Signs and symptoms
In humans, S. aureus is part of the normal microbiota present in the upper respiratory tract, and on skin and in the gut mucosa. S. aureus, along with similar species that can colonize and act symbiotically but can cause disease if they begin to take over the tissues they have colonized or invade other tissues, have been called "pathobionts".
After 72 hours, MRSA can take hold in human tissues and eventually become resistant to treatment. The initial presentation of MRSA is small red bumps that resemble pimples, spider bites, or boils; they may be accompanied by fever and, occasionally, rashes. Within a few days, the bumps become larger and more painful; they eventually open into deep, pus-filled boils. About 75 percent of CA-MRSA infections are localized to skin and soft tissue and usually can be treated effectively.
Risk factors
Some of the populations at risk:
- People with indwelling implants, prostheses, drains, and catheters
- People who are frequently in crowded places, especially with shared equipment and skin-to-skin contact
- People with weak immune systems (HIV/AIDS, lupus, or cancer sufferers; transplant recipients, severe asthmatics, etc.)
- Diabetics
- Intravenous drug users
- Users of quinolone antibiotics
- The elderly
- School children sharing sports and other equipment.
- College students living in dormitories
- People staying or working in a health care facility for an extended period of time
- People who spend time in coastal waters where MRSA is present, such as some beaches in Florida and the west coast of the United States
- People who spend time in confined spaces with other people, including occupants of homeless shelters, prison inmates, military recruits in basic training,
- Veterinarians, livestock handlers, and pet owners
- Those that ingest unpasteurized milk
- Those who are immunocompromised and also colonized
- Those with Chronic obstructive pulmonary disease
- Those who had thoracic surgery.
As many as 22% of those infected with MRSA do not have any discernable risk factors.
Hospitalized people
People who are hospitalized, including the elderly, are often immunocompromised and susceptible to infection of all kinds, including MRSA; when the infection is by MRSA this is called healthcare-associated or hospital-acquired methicillin-resistant Staphylococcus aureus (HA-MRSA). Generally, those infected by MRSA will stay infected for just under 10 days, if treated by a doctor, although effects may vary from person to person.
Surgical as well as nonsurgical wounds can be infected with HA-MRSA. Surgical site infections (SSI) occur on the skin surface but can spread to internal organs and blood to cause sepsis. Transmission occurs between healthcare providers and patients. This is because some providers may inconsistently neglect to perform hand-washing between examinations.
People in nursing homes are at risk for all the reasons above, further complicated by the generally weaker immune systems of the elderly or other residents in need of such care.
Prison inmates, military recruits
Prisons, and military barracks, can be crowded and confined, and poor hygiene practices may proliferate, thus putting inhabitants at increased risk of contracting MRSA. Cases of MRSA in such populations were first reported in the United States, and then in Canada. The earliest reports were made by the Center for Disease Control (CDC) in US state prisons. In the news media, hundreds of reports of MRSA outbreaks in prisons appeared between 2000 and 2008. For example, in February 2008, the Tulsa County jail in Oklahoma started treating an average of 12 S. aureus cases per month. A report on skin and soft tissue infections in the Cook County jail in Chicago in 2004-05 demonstrated MRSA was the most common cause of these infections among cultured lesions, and few risk factors were more strongly associated with MRSA infections than infections caused by methicillin-susceptible S. aureus. In response to these and many other reports on MRSA infections among incarcerated and recently incarcerated persons, the Federal Bureau of Prisons has released guidelines for the management and control of the infections, although few studies provide an evidence base for these guidelines. During a recent study in Fort Benning Georgia, a variety of military recruits both healthy and those suffering from soft tissue infections were tested for MRSA as well as other pathogens. The researchers determined that a significant portion of trainees were either asymptomatic carriers of MRSA or that MRSA was the cause of their infection.
Animals
Antibiotic use in livestock increases the risk that MRSA will develop among the livestock; strains MRSA ST 398 and CC398 are transmissible to humans. Generally, animals are asymptomatic.
Domestic pets are susceptible to MRSA infection from their owners; MRSA infected pets can also transmit MRSA to humans.
Athletes
Locker rooms, gyms, and related athletic facilities offer potential sites for MRSA contamination and infection. Athletes have been identified as a high risk group. A study linked MRSA to the abrasions caused by artificial turf. Three studies by the Texas State Department of Health found the infection rate among football players was 16 times the national average. In October 2006, a high-school football player was temporarily paralyzed from MRSA-infected turf burns. His infection returned in January 2007 and required three surgeries to remove infected tissue, as well as three weeks of hospital stay. In 2013, Lawrence Tynes, Carl Nicks, and Johnthan Banks of the Tampa Bay Buccaneers were diagnosed with MRSA. Tynes and Nicks apparently did not contract the infection from each other, but it is unknown if Banks contracted it from either individual. In 2015, Los Angeles Dodgers' infielder Justin Turner was infected while the team visited the New York Mets. In October 2015, New York Giants tight end Daniel Fells was hospitalized with a serious MRSA infection.
Children
MRSA is becoming a critical problem in pediatric settings; recent studies found 4.6% of patients in U.S. health-care facilities, (presumably) including hospital nurseries, were infected or colonized with MRSA. Children (and adults, as well) who come in contact with day-care centers, playgrounds, locker rooms, camps, dormitories, classrooms and other school settings, and gyms and workout facilities are at higher risk of getting MRSA. Parents should be especially cautious of children who participate in activities where sports equipment is shared, such as football helmets and uniforms.
Diagnosis
Diagnostic microbiology laboratories and reference laboratories are key for identifying outbreaks of MRSA. Normally, the bacterium must be cultured from blood, urine, sputum, or other body-fluid samples, and in sufficient quantities to perform confirmatory tests early-on. Still, because no quick and easy method exists to diagnose MRSA, initial treatment of the infection is often based upon 'strong suspicion' and techniques by the treating physician; these include quantitative PCR procedures, which are employed in clinical laboratories for quickly detecting and identifying MRSA strains.
Another common laboratory test is a rapid latex agglutination test that detects the PBP2a protein. PBP2a is a variant penicillin-binding protein that imparts the ability of S. aureus to be resistant to oxacillin.
Genetics
Antimicrobial resistance is genetically based; resistance is mediated by the acquisition of extrachromosomal genetic elements containing resistance genes. Examples include plasmids, transposable genetic elements, and genomic islands, which are transferred between bacteria through horizontal gene transfer. A defining characteristic of MRSA is its ability to thrive in the presence of penicillin-like antibiotics, which normally prevent bacterial growth by inhibiting synthesis of cell wall material. This is due to a resistance gene, mecA, which stops ?-lactam antibiotics from inactivating the enzymes (transpeptidases) critical for cell wall synthesis.
SCCmec
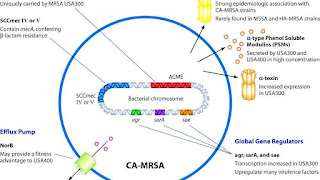
Staphylococcal cassette chromosome mec (SCCmec) is a genomic island of unknown origin containing the antibiotic resistance gene mecA. SCCmec contains additional genes beyond mecA, including the cytolysin gene psm-mec, which may suppress virulence in HA-acquired MRSA strains. In addition this locus encodes strain dependent gene regulatory RNA called psm-mecRNA. SCCmec also contains ccrA and ccrB; both genes encode recombinases that mediate the site-specific integration and excision of the SCCmec element from the S. aureus chromosome. Currently, six unique SCCmec types ranging in size from 21-67 kb have been identified; they are designated types I-VI and are distinguished by variation in mec and ccr gene complexes. Owing to the size of the SCCmec element and the constraints of horizontal gene transfer, a minimum of five clones are thought to be responsible for the spread of MRSA infections, with clonal complex (CC) 8 most prevalent. SCCmec is thought to have originated in the closely related Staphylococcus sciuri species and transferred horizontally to S. aureus.
Different SCCmec genotypes confer different microbiological characteristics, such as different antimicrobial resistance rates. Different genotypes are also associated with different types of infections. Types I-III SCCmec are large elements that typically contain additional resistance genes and are characteristically isolated from HA-MRSA strains. Conversely, CA-MRSA is associated with types IV and V, which are smaller and lack resistance genes other than mecA.
These distinctions were thoroughly investigated by Collins et al. in 2001 and can be explained by the fitness differences associated with carriage of a large or small SCCmec plasmid. Carriage of large plasmids, such as SCCmecI-III, is costly to the bacteria, resulting in compensatory decrease in virulence expression. MRSA is able to thrive in hospital settings with increased antibiotic resistance but decreased virulence- HA-MRSA targets immunocompromised, hospitalized hosts, thus a decrease in virulence is not maladaptive. In contrast, CA-MRSA tends to carry lower fitness cost SCCmec elements to offset the increased virulence and toxicity expression required to infect healthy hosts.
mecA
mecA is a biomarker gene responsible for resistance to methicillin and other ?-lactam antibiotics. After acquisition of mecA, the gene must be integrated and localized in the S. aureus chromosome. mecA encodes penicillin-binding protein 2a (PBP2a), which differs from other penicillin-binding proteins as its active site does not bind methicillin or other ?-lactam antibiotics. As such, PBP2a can continue to catalyze the transpeptidation reaction required for peptidoglycan cross-linking, enabling cell wall synthesis in the presence of antibiotics. As a consequence of the inability of PBP2a to interact with ?-lactam moieties, acquisition of mecA confers resistance to all ?-lactam antibiotics in addition to methicillin.
mecA is under the control of two regulatory genes, mecI and mecR1. MecI is usually bound to the mecA promoter and functions as a repressor. In the presence of a ?-lactam antibiotic, MecR1 initiates a signal transduction cascade that leads to transcriptional activation of mecA. This is achieved by MecR1-mediated cleavage of MecI, which alleviates MecI repression. mecA is further controlled by two co-repressors, BlaI and BlaR1. blaI and blaR1 are homologous to mecI and mecR1, respectively, and normally function as regulators of blaZ, which is responsible for penicillin resistance. The DNA sequences bound by MecI and BlaI are identical; therefore, BlaI can also bind the mecA operator to repress transcription of mecA.
Arginine catabolic mobile element
The arginine catabolic mobile element (ACME) is a virulence factor present in many MRSA strains but not prevalent in MSSA. SpeG-positive ACME compensates for the polyamine hypersensitivity of S. aureus and facilitates stable skin colonization, wound infection, and person-to-person transmission.
Strains
Acquisition of SCCmec in methicillin-sensitive staphylococcus aureus (MSSA) gives rise to a number of genetically different MRSA lineages. These genetic variations within different MRSA strains possibly explain the variability in virulence and associated MRSA infections. The first MRSA strain, ST250 MRSA-1 originated from SCCmec and ST250-MSSA integration. Historically, major MRSA clones: ST2470-MRSA-I, ST239-MRSA-III, ST5-MRSA-II, and ST5-MRSA-IV were responsible for causing hospital-acquired MRSA (HA-MRSA) infections. ST239-MRSA-III, known as the Brazilian clone, was highly transmissible compared to others and distributed in Argentina, Czech Republic, and Portugal.
In the UK, the most common strains of MRSA are EMRSA15 and EMRSA16. EMRSA16 has been found to be identical to the ST36:USA200 strain, which circulates in the United States, and to carry the SCCmec type II, enterotoxin A and toxic shock syndrome toxin 1 genes. Under the new international typing system, this strain is now called MRSA252. EMRSA 15 is also found to be one of the common MRSA strains in Asia. Other common strains include ST5:USA100 and EMRSA 1. These strains are genetic characteristics of HA-MRSA.
Community-acquired MRSA (CA-MRSA) strains emerged in late 1990 to 2000, infecting healthy people who had not been in contact with health care facilities. Researchers suggest that CA-MRSA did not evolve from the HA-MRSA. This is further proven by molecular typing of CA-MRSA strains and genome comparison between CA-MRSA and HA-MRSA, which indicate that novel MRSA strains integrated SCCmec into MSSA separately on its own. By mid 2000, CA-MRSA was introduced into the health care systems and distinguishing CA-MRSA from HA-MRSA became a difficult process. Community-acquired MRSA is more easily treated and more virulent than hospital-acquired MRSA (HA-MRSA). The genetic mechanism for the enhanced virulence in CA-MRSA remains an active area of research. Especially the Panton-Valentine leukocidin (PVL) genes are of interest because they are a unique feature of CA-MRSA.
In the United States, most cases of CA-MRSA are caused by a CC8 strain designated ST8:USA300, which carries SCCmec type IV, Panton-Valentine leukocidin, PSM-alpha and enterotoxins Q and K, and ST1:USA400. The ST8:USA300 strain results in skin infections, necrotizing fasciitis and toxic shock syndrome, whereas the ST1:USA400 strain results in necrotizing pneumonia and pulmonary sepsis. Other community-acquired strains of MRSA are ST8:USA500 and ST59:USA1000. In many nations of the world, MRSA strains with different predominant genetic background types have come to predominate among CA-MRSA strains; USA300 easily tops the list in the U.S. and is becoming more common in Canada after its first appearance there in 2004. For example, in Australia ST93 strains are common, while in continental Europe ST80 strains, which carry SCCmec type IV, predominate. In Taiwan, ST59 strains, some of which are resistant to many non-beta-lactam antibiotics, have arisen as common causes of skin and soft tissue infections in the community. In a remote region of Alaska, unlike most of the continental U.S., USA300 was found rarely in a study of MRSA strains from outbreaks in 1996 and 2000 as well as in surveillance from 2004-06.
A MRSA strain, CC398, is found in intensively reared production animals (primarily pigs, but also cattle and poultry), where it can be transmitted to humans as LA-MRSA (livestock-associated MRSA).
Prevention
Screening
In health care settings, isolating those with MRSA from those without the infection, is one method to prevent transmission. Rapid culture and sensitivity testing and molecular testing identifies carriers and reduces infection rates.
MRSA can be identified by swabbing the nostrils and isolating the bacteria found inside the nostrils. Combined with extra sanitary measures for those in contact with infected people, swab screening people admitted to hospitals has been found to be effective in minimizing the spread of MRSA in hospitals in the United States, Denmark, Finland, and the Netherlands.
Hand washing
The CDC offers suggestions for preventing the contraction and spread MRSA infection which are applicable to those in community settings, including incarcerated populations, childcare center employees, and athletes. To prevent the spread of MRSA the recommendations are to wash hands using soap and water or an alcohol-based sanitizer. Additional recommendations are to keep wounds clean and covered, avoid contact with other people's wounds, avoid sharing personal items such as razors or towels, shower after exercising at athletic facilities, and shower before using swimming pools or whirlpools.
In September 2004, the UK National Health Service announced its Clean Your Hands campaign. It was recommended that alcohol-based hand rubs be placed near beds to encourage staff to use the sanitizer more regularly. It was thought that even if this cuts infection by no more than 1%, the plan will pay for itself many times over.
Isolation
Excluding medical facilities, current US guidance does not require workers with MRSA infections to be routinely excluded from the general workplace. The National Institutes of Health recommends that those with wound drainage that cannot be covered and contained with a clean, dry bandage and those who cannot maintain good hygiene practices be reassigned. Workers with active infections are excluded from activities where skin-to-skin contact is likely to occur. To prevent the spread of staph or MRSA in the workplace, employers make available adequate facilities that encourage good hygiene. In addition, surface and equipment sanitizing conforms to the Environmental Protection Agency (EPA)-registered disinfectants. Health Departments recommend that preventing the spread of MRSA in the home can be to: launder materials that have come into contact with infected person separately and with a dilute bleach solution; reduce the bacterial load in your nose and on your skin; clean those things in the house that people regularly touch like sinks, tubs, kitchen counters, cell phones, light switches, doorknobs, phones, toilets, and computer keyboards.
Restricting antibiotic use
Glycopeptides, cephalosporins, and, in particular, quinolones are associated with an increased risk of colonisation of MRSA. Reducing use of antibiotic classes that promote MRSA colonisation, especially fluoroquinolones, is recommended in current guidelines.
Public health considerations
Mathematical models describe one way in which a loss of infection control can occur after measures for screening and isolation seem to be effective for years, as happened in the UK. In the "search and destroy" strategy that was employed by all UK hospitals until the mid-1990s, all hospitalized people with MRSA were immediately isolated, and all staff were screened for MRSA and were prevented from working until they had completed a course of eradication therapy that was proven to work. Loss of control occurs because colonised people are discharged back into the community and then readmitted; when the number of colonised people in the community reaches a certain threshold, the "search and destroy" strategy is overwhelmed. One of the few countries not to have been overwhelmed by MRSA is the Netherlands: An important part of the success of the Dutch strategy may have been to attempt eradication of carriage upon discharge from hospital.
The Centers for Disease Control and Prevention (CDC) estimated that about 1.7 million nosocomial infections occurred in the United States in 2002, with 99,000 associated deaths. The estimated incidence is 4.5 nosocomial infections per 100 admissions, with direct costs (at 2004 prices) ranging from $10,500 (£5300, EUR8000 at 2006 rates) per case (for bloodstream, urinary tract, or respiratory infections in immunocompetent people) to $111,000 (£57,000, EUR85,000) per case for antibiotic-resistant infections in the bloodstream in people with transplants. With these numbers, conservative estimates of the total direct costs of nosocomial infections are above $17 billion. The reduction of such infections forms an important component of efforts to improve healthcare safety. (BMJ 2007) MRSA alone was associated with 8% of nosocomial infections reported to the CDC National Healthcare Safety Network from January 2006 to October 2007.
This problem is not unique to one country; the British National Audit Office estimated that the incidence of nosocomial infections in Europe ranges from 4% to 10% of all hospital admissions. As of early 2005, the number of deaths in the United Kingdom attributed to MRSA has been estimated by various sources to lie in the area of 3,000 per year. Staphylococcus bacteria account for almost half of all UK hospital infections. The issue of MRSA infections in hospitals has recently been a major political issue in the UK, playing a significant role in the debates over health policy in the United Kingdom general election held in 2005.
Worldwide, an estimated 2 billion people carry some form of S. aureus; of these, up to 53 million (2.7% of carriers) are thought to carry MRSA. In the United States, 95 million carry S. aureus in their noses; of these, 2.5 million (2.6% of carriers) carry MRSA. A population review conducted in three U.S. communities showed the annual incidence of CA-MRSA during 2001-2002 to be 18-25.7/100,000; most CA-MRSA isolates were associated with clinically relevant infections, and 23% of people required hospitalization.
Decolonization
As of 2013 there had been no randomized clinical trials conducted to understand how to treat non-surgical wounds that had been colonized, but not infected, with MRSA, and insufficient studies had been conducted to understand how to treat surgical wounds that had been colonized with MRSA. As of 2013 it was not known whether strategies to eradicate MRSA colonization of people in nursing homes reduced infection rates.
Care should be taken when trying to drain boils, as disruption of surrounding tissue can lead to larger infections, or even infection of the blood stream (often with fatal consequences).
Mupirocin (Bactroban) 2% ointment can be effective at reducing the size of lesions. A secondary covering of clothing is preferred. As shown in an animal study with diabetic mice, the topical application of a mixture of sugar (70%) and 3% povidone-iodine paste is an effective agent for the treatment of diabetic ulcers with MRSA infection.
In the hospital setting toilet seats are a common vector for infection, and wiping seats clean before and/or after use can help to prevent the spread of MRSA. Door handles, faucets, light switches, etc. can be disinfected regularly with disinfectant wipes. Spray disinfectants can be used on upholstery. Carpets can be washed with disinfectant, and hardwood floors can be scrubbed with diluted tea tree oil (e.g. Melaleuca). Laundry soap containing tea tree oil may be effective at decontaminating clothing and bedding, especially if hot water and heavy soil cycles are used, however tea tree oil may cause a rash which MRSA can re-colonize. Alcohol-based sanitizers can be placed near bedsides, near sitting areas, in vehicles etc. to encourage their use.
Doctors may also prescribe antibiotics such as clindamycin, doxycycline, or trimethoprim/sulfamethoxazole.
Community settings
It may be difficult for people to maintain the necessary cleanliness if they do not have access to facilities such as public toilets with handwashing facilities. In the United Kingdom, the Workplace (Health, Safety and Welfare) Regulations 1992 requires businesses to provide toilets for their employees, along with washing facilities including soap or other suitable means of cleaning. Guidance on how many toilets to provide and what sort of washing facilities should be provided alongside them is given in the Workplace (Health, Safety and Welfare) Approved Code of Practice and Guidance L24, available from Health and Safety Executive Books. But there is no legal obligation on local authorities in the United Kingdom to provide public toilets, and although in 2008 the House of Commons Communities and Local Government Committee called for a duty on local authorities to develop a public toilet strategy this was rejected by the Government.
Agriculture
Some advocate regulations on the use of antibiotics in animal food to prevent the emergence of drug resistant strains of MRSA. MRSA is established in animals.
Treatment
Medication
Treatment is urgent and delays can be fatal. The location and history related to the infection determines the treatment. The route of administration of an antibiotic varies. Antibiotics effective against MRSA can be given by IV, oral, or a combination of both and depends on the specific circumstances and patient characteristics. The use of concurrent treatment with vancomycin other beta-lactam agents may have a synergistic effect.
Both CA-MRSA and HA-MRSA are resistant to traditional anti-staphylococcal beta-lactam antibiotics, such as cephalexin. CA-MRSA has a greater spectrum of antimicrobial susceptibility to sulfa drugs (like co-trimoxazole (trimethoprim/sulfamethoxazole), tetracyclines (like doxycycline and minocycline) and clindamycin (for osteomyelitis). MRSA can be eradicated with a regimen of linezolid, though treatment protocols vary and serum levels of antibiotics vary widely person to person and may affect outcomes. The effective treatment of MRSA with linezolid has been successful in 87% of people. Linezolid is more effective in soft tissue infections than vancomycin. This is compared to eradication of infection in those with MRSA treated with vancomycin. Treatment with vancomycin is successful in approximately 49% of people. Linezolid and daptomycin belong to the newer oxazolidinones which has been shown to be effective against both CA-MRSA and HA-MRSA. The Infectious Disease Society of America recommends vancomycin, linezolid, or clindamycin (if susceptible) for treating those with MRSA pneumonia. Ceftaroline, a fifth-generation cephalosporin, is the first beta-lactam antibiotic approved in the US to treat MRSA infections in skin and soft tissue or community acquired pneumonia.
Vancomycin and teicoplanin are glycopeptide antibiotics used to treat MRSA infections. Teicoplanin is a structural congener of vancomycin that has a similar activity spectrum but a longer half-life. Because the oral absorption of vancomycin and teicoplanin is very low, these agents can be administered intravenously to control systemic infections. Treatment of MRSA infection with vancomycin can be complicated, due to its inconvenient route of administration. Moreover, the efficacy of vancomycin against MRSA is inferior to that of anti-staphylococcal beta-lactam antibiotics against methicillin-susceptible Staphylococcus aureus (MSSA).
Several newly discovered strains of MRSA show antibiotic resistance even to vancomycin and teicoplanin. These new strains of the MRSA bacterium have been dubbed vancomycin intermediate-resistant Staphylococcus aureus (VISA). Linezolid, quinupristin/dalfopristin, daptomycin, ceftaroline, and tigecycline are used to treat more severe infections that do not respond to glycopeptides such as vancomycin. Current guidelines recommend daptomycin for VISA bloodstream infections and endocarditis.
Developments
This left vancomycin as the only effective agent available at the time. However, strains with intermediate (4-8 ?g/ml) levels of resistance, termed glycopeptide-intermediate Staphylococcus aureus (GISA) or vancomycin-intermediate Staphylococcus aureus (VISA), began appearing in the late 1990s. The first identified case was in Japan in 1996, and strains have since been found in hospitals in England, France and the US. The first documented strain with complete (>16 ?g/ml) resistance to vancomycin, termed vancomycin-resistant Staphylococcus aureus (VRSA) appeared in the United States in 2002. However, in 2011, a variant of vancomycin has been tested that binds to the lactate variation and also binds well to the original target, thus reinstating potent antimicrobial activity.
A new class of antibiotics, oxazolidinones, became available in the 1990s, and the first commercially available oxazolidinone, linezolid, is comparable to vancomycin in effectiveness against MRSA. Linezolid resistance in S. aureus was reported in 2001, but infection rates have been at consistently low levels and in the United Kingdom and Ireland, no resistance was found in staphylococci collected from bacteremia cases between 2001 and 2006.
Community-acquired MRSA (CA-MRSA) has now emerged as an epidemic that is responsible for rapidly progressive, fatal diseases, including necrotizing pneumonia, severe sepsis, and necrotizing fasciitis. MRSA is the most frequently identified antimicrobial drug-resistant pathogen in US hospitals. The epidemiology of infections caused by MRSA is rapidly changing. Since 2000, infections caused by this organism have emerged in the community. The two MRSA clones in the United States most closely associated with community outbreaks, USA400 (MW2 strain, ST1 lineage) and USA300, often contain Panton-Valentine leukocidin (PVL) genes and, more frequently, have been associated with skin and soft tissue infections. Outbreaks of CA-MRSA infections have been reported in correctional facilities, among athletic teams, among military recruits, in newborn nurseries, and among men that have sex with men. CA-MRSA infections now appear endemic in many urban regions and cause most CA-S. aureus infections.
Skin and soft-tissue infections
In skin abscesses, the primary treatment recommended is removal of dead tissue, incision and drainage. More data is needed to determine the effectiveness of specific antibiotics therapy in SSIs. Examples of soft tissue infections from MRSA include: ulcers, impetigo, abscesses, and surgical site infections.
Children
In skin infections and in secondary infection sites topical mupirocin is used successfully. For bacteremia and endocarditis, vancomycin or daptomycin is considered. For children with MRSA infected bone or joints, treatment is individualized and long-term. Neonates can develop Neonatal pustulosis as a result of topical infection with MRSA. Clindamycin is not approved for the treatment of MRSA infection it is still used in children for soft tissue infections.
Endocarditis and bacteremia
Evaluation for the replacement of a prosthetic valve is considered. Appropriate antibiotic therapy may be administered for up to six weeks. Four to six weeks of antibiotic treatment is often recommended, and is dependent upon the extent of MRSA infection.
Respiratory infections
CA-MRSA in hospitalized patients pneumonia treatment begins before culture results. After the susceptibility to antibiotics is performed, the infection may be treated with vancomycin or linezolid for up to 21 days. If the pneumonia is complicated by the accumulation of pus in the pleural cavity surrounding the lungs, drainage may be done along with antibiotic therapy. People with cystic fibrosis may develop respiratory complications related to MRSA infection. The incidence of MRSA in those with cystic fibrosis increased during 2000 to 2015 by five times. Most of these infections were HA-MRSA. MRSA accounts for 26% of lung infections in those with cystic fibrosis.
Bone and joint infections
Cleaning the wound of dead tissue and draining abscesses is the first action to treat the MRSA infection. Administration of antibiotics is not standardized and is adapted by a case-by-case basis. Antibiotic therapy can last up to 1 to 3 months and sometimes even longer.
Infected implants
MRSA infection can occur associated with implants and joint replacements. Recommendations on treatment are based upon the length of time the implant has been in place. In cases of a recent placement of a surgical implant or artificial joint, the device may be retained while antibiotic therapy continues. If the placement of the device has occurred over 3 weeks ago, the device may be removed. Antibiotic therapy is used in each instance sometimes long-term.
Central nervous system
MRSA can infect the central nervous system and form brain abscess, subdural empyema, and spinal epidural abscess. Excision and drainage can be done along with antibiotic treatment. Septic thrombosis of cavernous or dural venous sinus can sometimes be a complication.
Other infections
Treatment is not standardized for other instances of MRSA infection in a wide range of tissues. Treatment varies for MRSA infections related to: subperiosteal abscesses, necrotizing pneumonia, cellulitis, pyomyositis, necrotizing fasciitis, mediastinitis, myocardial, perinephric, hepatic, and splenic abscesses, septic thrombophlebitis, and severe ocular infections, including endophthalmitis. Pets can be reservoirs and pass on MRSA to people. In some cases, the infection can be symptomatic and the pet can suffer a MRSA infection. Health departments recommend that the pet be taken to the veterinarian if MRSA infections keep occurring in the people who have contact with the pet.
Epidemiology
HA-MRSA
In a US cohort study of 1300 healthy children, 2.4% carried MRSA in their nose. Bacterial sepsis occurs with most (75%) of cases of invasive MRSA infection. In 2009, there were an estimated 463,017 hospitalization due to MRSA or a rate of 11.74 per 1,000 hospitalizations. Many of these infections are less serious, but the Centers for Disease Control and Prevention (CDC) estimates that there are 80,461 invasive MRSA infections and 11,285 deaths due to MRSA annually. In 2003, the cost for a hospitalization due to a MRSA was $92,363. A hospital stay for MSSA was $52,791 (USD).
Infection after surgery is relatively uncommon, but occurs as much as 33% in specific types of surgeries. Infections of surgical sites range from 1% to 33%. MRSA sepsis that occurs within 30 days has a 15-38% mortality rate. MRSA sepsis that occurs within one year following a surgical infection has a mortality rate of around 55%. There may be increased mortality associated with cardiac surgery. There is a rate of 12.9% in those infected with MRSA while only a 3% infected with other organisms. SSIs infected with MRSA had longer hospital stays than those who did not.
Globally, MRSA infection rates are dynamic and vary year to year. According to The 2006 SENTRY Antimicrobial Surveillance Program report, the incidence of MRSA blood stream infections was 35.9 per cent in North America. MRSA blood infections in Latin America was 29%. European incidence was 22.8%. The rate of all MRSA infections in Europe ranged from 50% per cent in Portugal down to 0.8 per cent in Sweden. Overall MRSA infection rates varied in Latin America: Colombia and Venezuela combined had 3%, Mexico had 50%, Chile 38%, Brazil 29%, and Argentina 28%.
CA-MRSA
In a US cohort study of 1300 healthy children, 2.4% carried MRSA in their nose.
There are concerns that the presence of MRSA in the environment may allow resistance to be transferred to other bacteria through phages (viruses that infect bacteria). The source of MRSA could come from hospital waste, farm sewage, and other waste water.
LA-MRSA
Livestock associated MRSA (LA-MRSA) has been observed in Korea, Brazil, Switzerland, Malaysia, India, Great Britain, Denmark, and China.
History
A 1,000-year-old eye salve recipe found in the medieval Bald's Leechbook at the British Library, one of the earliest known medical textbooks, was found to have activity against MRSA in vitro and in skin wounds in mice.
MRSA was first discovered in 1961 In 1959 - 1961. In 1961, Methicillin was licensed in England to treat penicillin-resistant S. aureus infections. In 1961 the first known MRSA isolates were reported in a British study, and from 1961 to 1967 there were infrequent hospital outbreaks in Western Europe and Australia. Other reports of MRSA began to be described in the 1970s. Resistance to other antibiotics was documented in some strains of S. aureus. In 1996, vancomycin resistance was reported in Japan. In many countries, outbreaks of MRSA infection was reported to be transmitted between hospitals. The rate had increased to 22% by 1995, and by 1997 the percent of hospital S. aureus infections attributable to MRSA had reached 50%.
The first report of community-associated MRSA (CA-MRSA) occurred in 1981, and in 1982 there was a large outbreak of CA-MRSA among intravenous drug users in Detroit, Michigan. Additional outbreaks of CA-MRSA were reported through the 1980s and 1990s, including outbreaks among Australian Aboriginal populations that had never been exposed to hospitals. In the mid-1990s there were scattered reports of CA-MRSA outbreaks among US children. While HA-MRSA rates stabilized between 1998 and 2008, CA-MRSA rates continued to rise. A report released by the University of Chicago Children's Hospital comparing two time periods (1993-1995 and 1995-1997) found a 25-fold increase in the rate of hospitalizations due to MRSA among children in the United States. In 1999 the University of Chicago reported the first deaths from invasive MRSA among otherwise healthy children in the United States. By 2004, the genome for various strains of MRSA were described.
It has been argued that the observed increased mortality among MRSA-infected people may be the result of the increased underlying morbidity of these people. Several studies, however, including one by Blot and colleagues, that have adjusted for underlying disease still found MRSA bacteremia to have a higher attributable mortality than methicillin-susceptible S. aureus (MSSA) bacteremia.
A population-based study of the incidence of MRSA infections in San Francisco during 2004-05 demonstrated that nearly 1 in 300 residents suffered from such an infection in the course of a year and that greater than 85% of these infections occurred outside of the healthcare setting. A 2004 study showed that people in the United States with S. aureus infection had, on average, three times the length of hospital stay (14.3 vs. 4.5 days), incurred three times the total cost ($48,824 vs. $14,141), and experienced five times the risk of in-hospital death (11.2% vs 2.3%) than people without this infection. In a meta-analysis of 31 studies, Cosgrove et al., concluded that MRSA bacteremia is associated with increased mortality as compared with MSSA bacteremia (odds ratio= 1.93; 95% CI = 1.93 ± 0.39). In addition, Wyllie et al. report a death rate of 34% within 30 days among people infected with MRSA, a rate similar to the death rate of 27% seen among MSSA-infected people.
In the US, the Centers for Disease Control and Prevention issued guidelines on October 19, 2006, citing the need for additional research, but declined to recommend such screening.
According to the CDC, the most recent estimates of the incidence of healthcare-associated infections that are attributable to MRSA in the United States indicate a decline in such infection rates. Incidence of MRSA central line-associated blood stream infections as reported by hundreds of intensive care units decreased 50-70% from 2001-2007. A separate system tracking all hospital MRSA bloodstream infections found an overall 34% decrease between 2005-2008.
In 2010, vancomycin was the drug of choice.
Across Europe, based mostly on data from 2013 seven countries (Iceland, Norway, Sweden, Netherlands, Denmark, Finland, and Estonia, from lowest to higher) had low levels of hospital-acquired MRSA infections compared to the others, and among countries with higher levels significant improvements had been made only in Bulgaria, Poland and the British Isles.
Research
Various antibacterial chemical extracts from various species of the Sweetgum tree, (genus Liquidambar) have been investigated for their activity in inhibiting MRSA. Specifically these are: cinnamic acid, cinnamyl cinnamate, ethyl cinnamate, benzyl cinnamate, styrene, vanillin, cinnamyl alcohol, 2-phenylpropyl alcohol, 3-phenylpropyl cinnamate, and vanillin.
The delivery of inhaled antibiotics along with systematic administration to treat MRSA are being developed. It's believed that this will improve the outcomes of those with cystic fibrosis and other respiratory infections.
Additional images
In popular culture
MRSA is frequently a media topic, especially if well-known personalities have announced that they have or have had the infection. Outbreaks of infection appear regularly in newspapers and television news programs. A report on skin and soft tissue infections in the Cook County jail in Chicago in 2004-05 demonstrated MRSA was the most common cause of these infections among those incarcerated there. Lawsuits that are filed against those who are accused of infecting others with MRSA are also popular stories in the media. MRSA will be included in experiments and cultured on the International Space Station to observe the effects of zero gravity on its evolution.
National Public Radio broadcast an episode of Fresh Air with MRSA as the topic. MRSA is the topic of television shows books and movies.
See also
- Antimicrobial resistance
- Carbapenem-resistant enterobacteriaceae
- Medications used to treat MRSA
- Teixobactin
- XF-73
References
Further reading
- The Centers for Disease Control and Prevention information, prevention, statistics, at risk groups, causes, educational resources, and environmental factors.
- National Institute for Occupational Safety and Health information on the bacteria, exposure in the workplace, and reducing risks of being infected.
- Medline research, information, treatments, and causes.
- Maryn McKenna (2011). Superbug: The Fatal Menace of MRSA. Free Press. ISBN 978-1-4165-5728-9.
- Karch, Amy (2017). Focus on nursing pharmacology. Philadelphia: Wolters Kluwer. ISBN 9781496318213.
- Vallerand, April (2017). Davis's drug guide for nurses. Philadelphia: F.A. Davis Company. ISBN 9780803657052.
Source of the article : Wikipedia